Preparation of Haloarenes
1. By electrophilic substitution reaction
Chemical Reactions of Haloarenes
1. By Nucleophilic substitution reaction
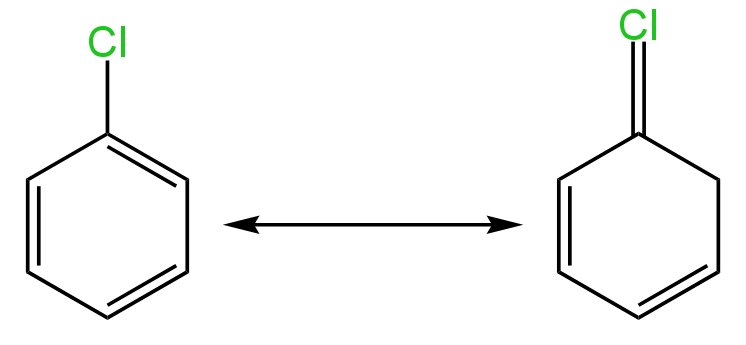
Aryl halides are less reactive toward Nucleophilic substitution reaction due to resonance effect.
Here C-Cl bond acquires a partial bond character due to resonance has a result in of this bond breaking in Haloarenes is difficult then in haloalkanes therefore Haloarenes are less reacted towards nucleophilic substitution reaction.
1. Instability of Carbonation
Haloarenes does not follow SN¹ mechanism to form phenyl Carbonation because it is unstable in nature.
2. Electro Static Repulsion
Since nucleophile is electron rich in nature it does not combines with electron rich benzeen ring due to electrostatic repulsion between.
2. Electrophilic Substitution Reaction
Halogens are para and ortho directing for electrophilic substitutions.
Electron density is more at ortho and Para position so attacking of nucleophile always take place on this places.